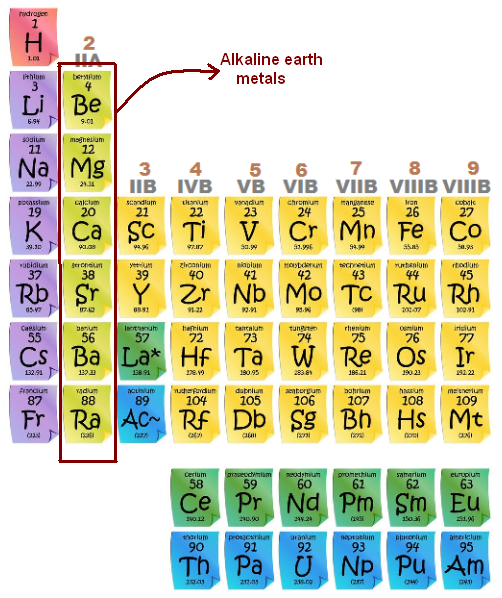
Which Region Contains the Alkaline Earth Metal Family of Elements
The alkaline earth metal family of elements is found in the second region of the periodic table. This region includes elements with atomic numbers from 4 to 12. The alkaline earth metals are beryllium, magnesium, calcium, strontium, barium, and radium.
These elements are all silver-colored metals that are relatively soft and have a low density. The alkaline earth metals are all reactive and do not occur naturally in their elemental form.
The alkaline earth metal family of elements includes beryllium, magnesium, calcium, strontium, barium, and radium. These elements are found in the second column of the periodic table and share many similarities. They are all silvery-white in color and have high melting and boiling points.
Additionally, they are all reactive to water and air. The most notable difference between the alkaline earth metals is their reactivity. Beryllium is the least reactive while radium is the most reactive.
This is due to differences in their valence electrons. The outermost orbital of beryllium only contains two valence electrons while radium contains eight valence electrons. The more valence electrons an element has, the more likely it is to form compounds with other elements.
Which Region Contains the Noble Gases?
Most of the periodic table is divided into four sections, or regions. These include the s-block, p-block, d-block, and f-block. The noble gases are located in the far right column of the periodic table in Group 18.
This region is also referred to as the zero groups because it contains elements with an electronic configuration of ns2 np6. The noble gases are all nonmetals and are extremely stable due to their full valence shells. The first element in this region is helium, which was discovered in 1868 by French astronomer Pierre Janssen.
Helium is the second lightest element and is used in a variety of applications including balloons, blimps, and welding torches. The next element is neon, which is used in neon lights and lasers. Argon is the third element and it makes up approximately 1% of Earth’s atmosphere.
Krypton and xenon are both used in fluorescent lights and photographic flashbulbs. Finally, radon is a radioactive gas that occurs naturally from the decay of uranium minerals in rocks and soil. The noble gases are all odorless, colorless, and tasteless.
They are also nonreactive under most conditions due to their full valence shells.
Credit: quizlet.com
Which Region Or Regions of the Periodic Table are the Alkaline Earth Metals?
The alkaline earth metals are a group of elements in the periodic table. They include beryllium, magnesium, calcium, strontium, barium, and radium. These elements are all metallic and have high melting points.
They are also highly reactive, so they are not found in nature as pure elements. Instead, they are found in minerals or compounds. The alkaline earth metals get their name from their chemical properties.
When they react with water, they form bases (alkalis) that neutralize acids. Beryllium is the lightest of the alkaline earth metals and is used in aerospace applications and nuclear reactors. Magnesium is the next lightest metal and is used in construction and transportation applications.
Calcium is the heaviest metal in this group and is an important component of bones and teeth. Strontium and barium are radioactive elements that are used in research applications. Radium is also radioactive and was once used in cancer treatment but has since been replaced by safer alternatives.
Where are the Alkaline Earth Metals Found?
The alkaline earth metals are a group of six elements in the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The name “alkaline earth” comes from its reactivity with acids.
All of the alkaline earth metals are found in nature, but not in large quantities. Beryllium is the least abundant, and radium is the most abundant. Most of these elements are found in minerals, but some can be found in other forms.
For example, calcium can be found in limestone or gypsum. These elements have a number of uses. Beryllium is used in alloys and ceramics, for example.
Magnesium is used in explosives and flares. Calcium is important for bone health, and strontium is used in nuclear reactors to absorb neutrons. Barium has a number of medical uses, such as contrast agents for X-rays. Radium was once used to make self-luminous paints, but this use has declined due to its radioactivity.
Which Family Are the Alkaline Metals?
Alkaline metals are a group of chemical elements in the periodic table. They are all shiny, silver-colored metals with low densities, low melting points, and high boiling points. The alkaline metals include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr).
All of these elements have very similar properties, due to their similar electronic structures. The name “alkaline metal” comes from the fact that these elements all form hydroxides – compounds with an OH group – when they react with water. These hydroxides are all basic, meaning they neutralize acids to form salt and water.
The alkaline metals are found in Group 1 of the periodic table. This means they have one valence electron in their outermost energy level – which is why they’re so reactive. When these elements lose that one electron, they become positively charged ions called cations.
Lithium is the lightest of the alkaline metals and has the smallest atomic radius. It’s also the least reactive element in this group – meaning it doesn’t form as many compounds as the other elements do. Sodium is next on the list, followed by potassium, rubidium, cesium, and francium.
Francium is actually so reactive that it’s rarely found in nature – most of its isotopes have short half-lives and decay quickly into other elements. All of the alkaline metals are used in a variety of ways: * Lithium is used in batteries, glassmaking, and medicine;
* Sodium chloride (table salt) is obtained from sodium;
* Potassium is used in agriculture and medicine;
* Rubidium has a wide range of applications including lasers and photocells;
* Cesium is used as a coolant for nuclear reactors;
Families of Elements
Conclusion
The alkaline earth metal family of elements includes beryllium, magnesium, calcium, strontium, barium, and radium. These elements are found in the second row of the periodic table. They are all metals with shiny surfaces.
Alkaline earth metals have many uses. Beryllium is used in making nuclear weapons and in X-ray machines. Magnesium is used in making car parts and airplane parts.
Calcium is an important part of our diet and is also used in making cement. Strontium is used to make fireworks and glass. Barium is used to make X-rays and rat poison.
Radium was once used to make watch dials glow in the dark but it is now considered too dangerous to use because it is radioactive.